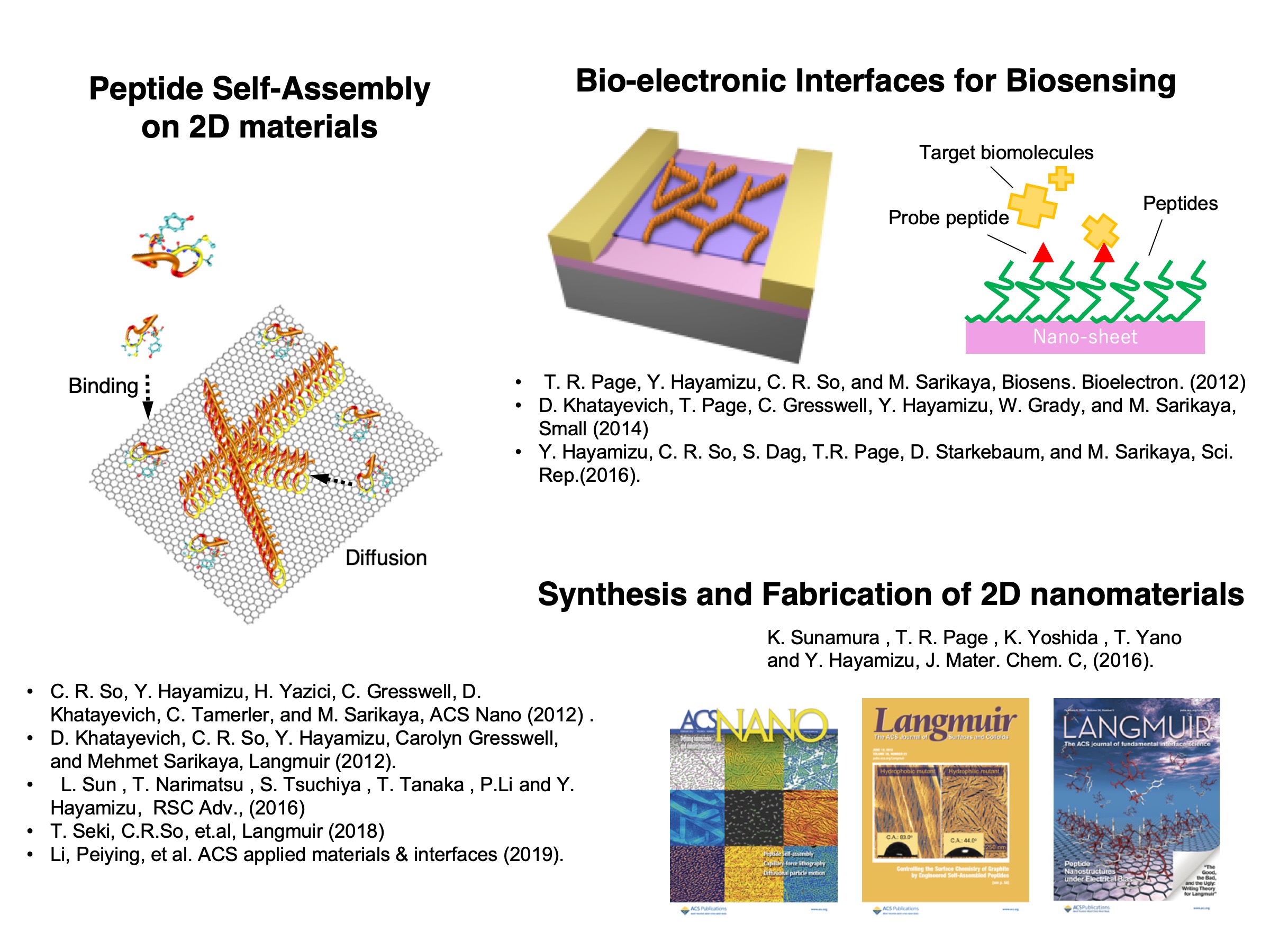
We are interested in interfaces between biological materials and inorganic surfaces. Especially, we employ two-dimensional (2D) nanosheets as the inorganic surface because of their unique physical properties. In our previous researches, we have developed several types of peptides which have abilities to form ordered nanostructures on those 2D nanosheets, and understood the mechanism of their interactions in the manner of self-assembly and (opto-)electronics.
Peptide Self-Assembly on 2D materials
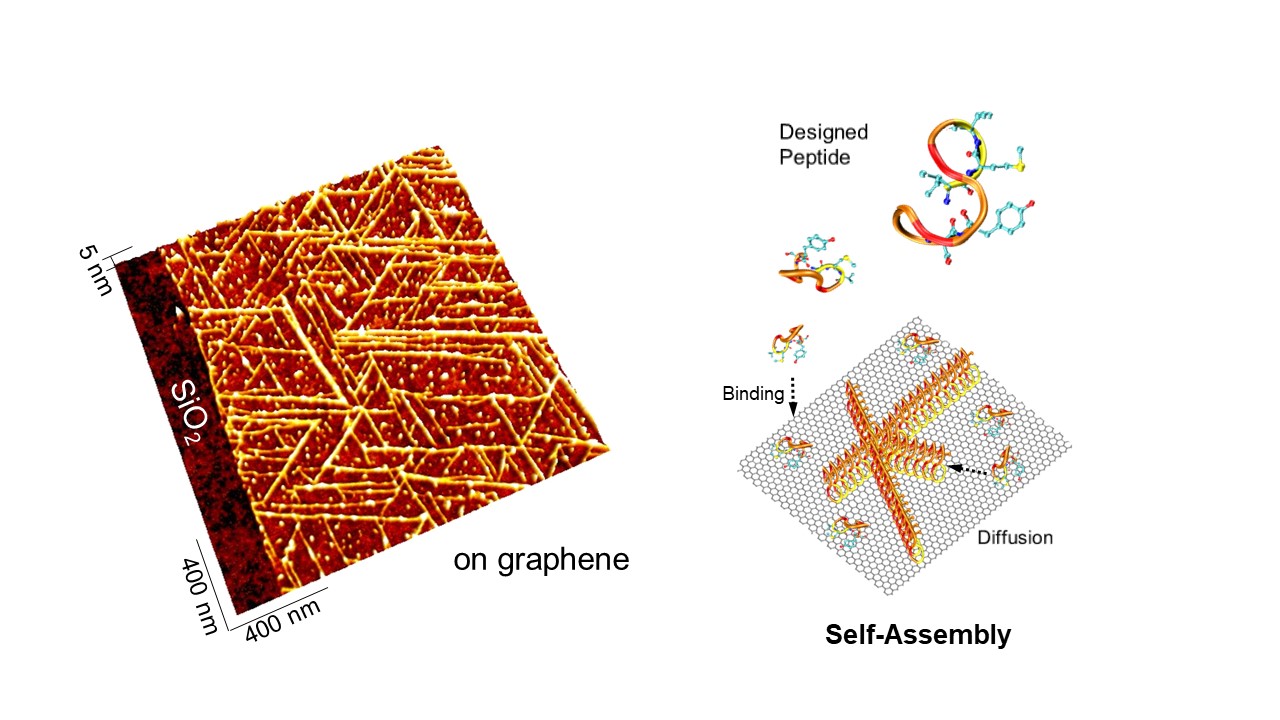
Desinged peptides spontaneously organize into long-range ordered structures on 2D nanomaterials such as graphene. Peptides in solution first bind to the surface. Next, peptides diffuse on the surface to find each other. Then, they assemble into organized structure. The peptide has tyrosine, aromatic amino acid. Due to the pi-pi interacton, the peptide may stick on the graphene surface. In fact, the peptide forms nanowires on the single-layer graphene as shown in the image of atomic force microscope. Interestingly, there is no peptides found on silicon dioxide surface. It indicates the specific peptide binding to the graphene surface.
Modulation of Electronic states of 2D materials
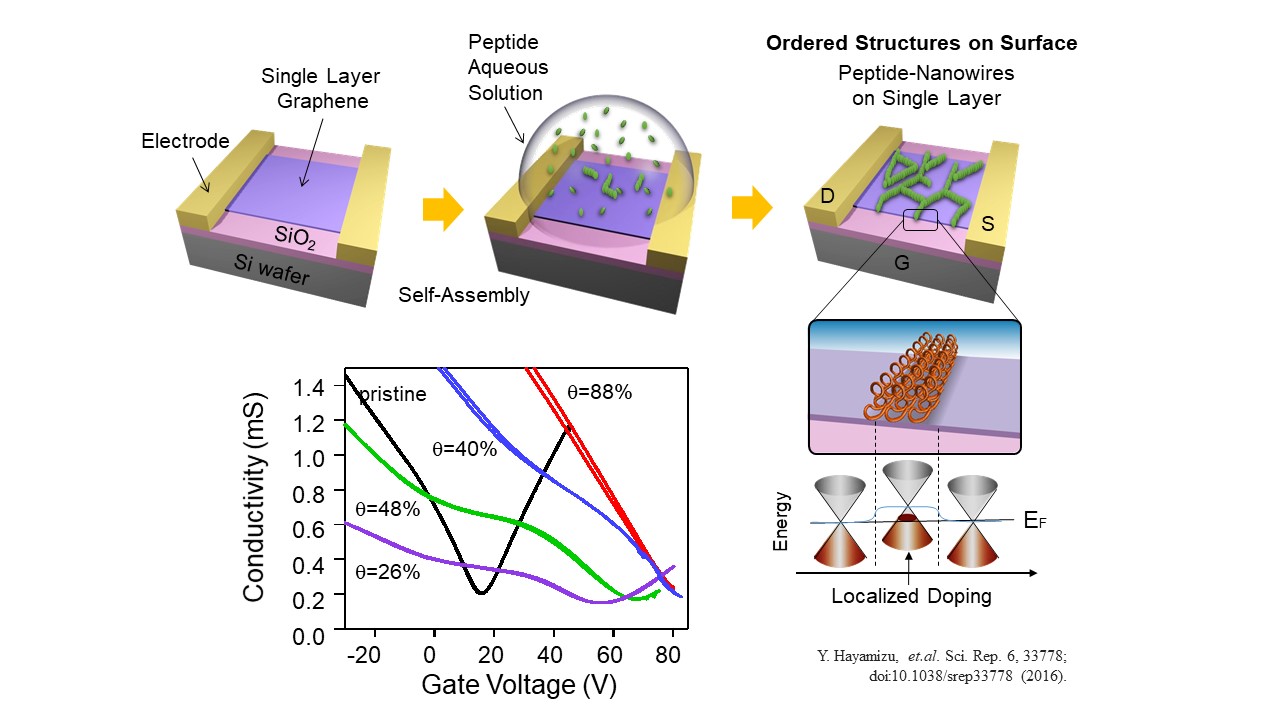
The self-assembled peptides modulated the electrical properties of graphene. The conductivity of graphene over varios gate-volatage showed the single conductivity minimum, charge neutral point (CNP), in the case of pristine graphene field effect transistor. But, once peptides self-assembled on the surface, there were a new minimum found at the higher voltage.As increasing the coverage of the peptides, the minimau at the low voltage gradually disappeared. It indicates that the organized peptides formed a spatial modulation of the charge density in the graphene.More importantly, the high overage of pepitdes provide a same transistor mobility (slope of the curve) as the pristine graphene, implying that peptides can act as a molecular scaffold for biosensing without degradation of the intrinsic graphene electrical properties.
Y. Hayamizu et.al., Scientific Reports volume6, Article number: 33778 (2016)
Peptides like silk protein for stable ordered structures on two-dimensional materials
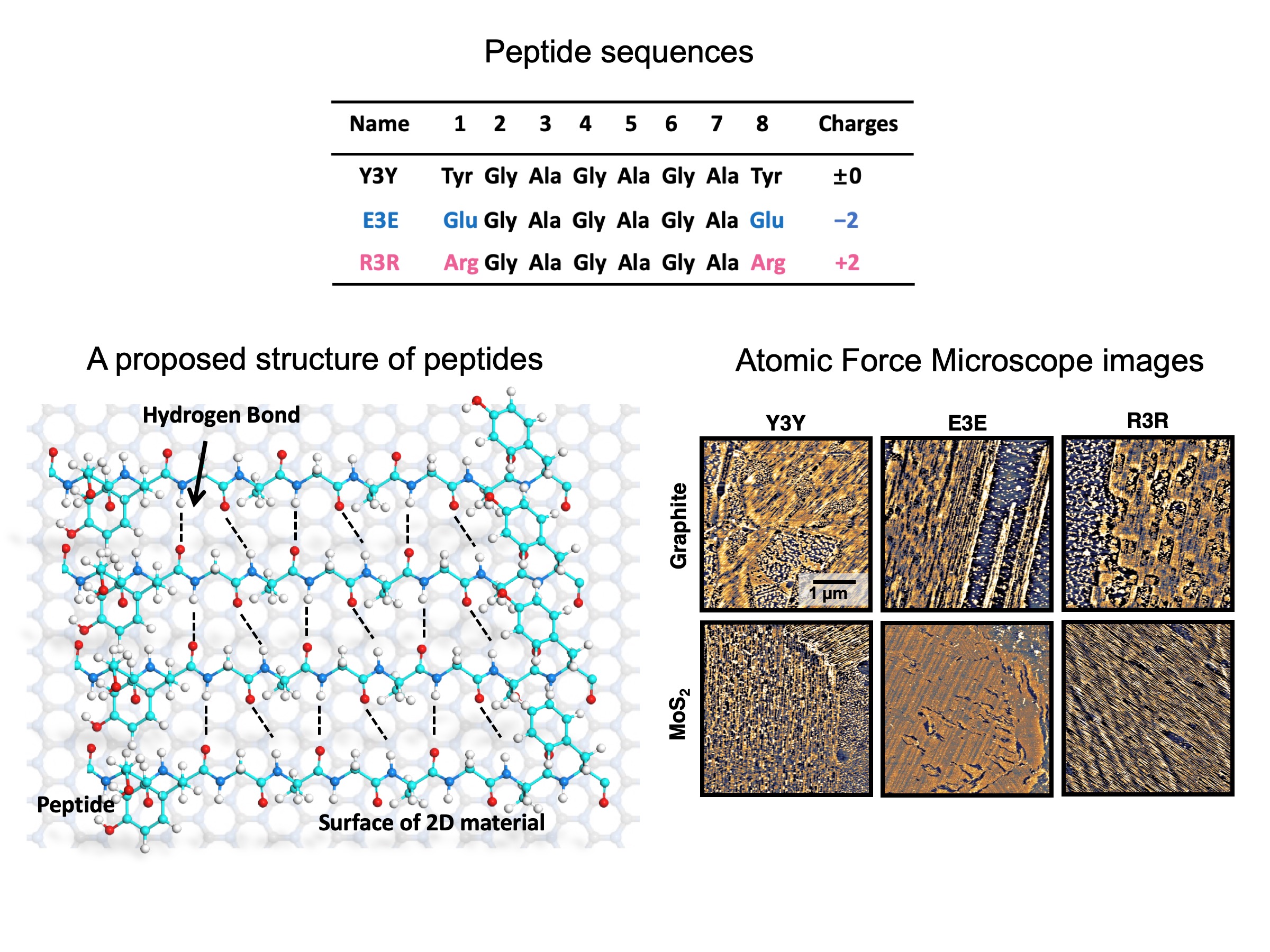
The highly aligned peptides on the two-dimensional materials can be used as a molecular scaffold because of its high bio-compatibility and high structural uniformity. Here, one of the challeges in the self-assembled peptides in the usage for biosensoring is the structural stability. The molecular scaffold needs to be stable so that the bio-probes to catch the target biomolecules can be immobilized on the surface stably. However, some of self-assembled peptides do not persist their ordered structures under electrolyte solutions. To overcome this problem, we established a new series of peptides, which have an ability to form ordered structures on the two-dimensional nanomaterials and persist their self-assembled structures under biosensing circumstances.These peptides have a repreated sequence of Glysine and Alanine by mimicking the amino acid sequence of silk protein, fibroin. These peptides revealed nicely ordered structures on graphene and MoS2, and high structural stability under water or electrolyte solutions, even under applied electrochemical bias.
P. Li, K. Sakuma, S. Tsuchiya, L. Sun, and Y. Hayamizu, ACS Applied Materials & Interfaces 2019 11 (23), 20670-20677



